More than 300 Modifications
How do we modify your Oligos?
Oligonucleotides can be modified by direct incorporation during the synthesis or by post-synthesis labelling.
Direct Incorporation
3' modifications
3’ modifications are only possible if the corresponding solid support (CPG column) is available as the automated oligonucleotide synthesis is realised from 3’to 5’.
In addition the modification must be compatible with the chemistries used during the synthesis.
Typical examples are 3’-phosphate, 3’ Biotin, 3’ FAM, 3’ DDQ I, 3’ BHQ-1®…
5’ and internal modifications
Many modifications can be directly introduced at the 5’ end or at internal positions of the oligonucleotides using the phosphoramidites.
However these modifications need to support the somewhat harsh cleavage-deprotection conditions including a strong basic pH.
Typical examples are 5’ Biotin, 5’ Phosphate, 5’Cholesterol, 5’ FAM, 8-Oxo-dA, Biotin-dT, DABCYL-dT…
Post-synthesis incorporation
Post-synthesis modifications may influence the yield of the reaction. A lower yield may result from poly-modifications and/or strong secondary structures.
Two major post-synthesis reactions are used to introduce sensitive dyes or compounds that do not exist as phosphoramidites.
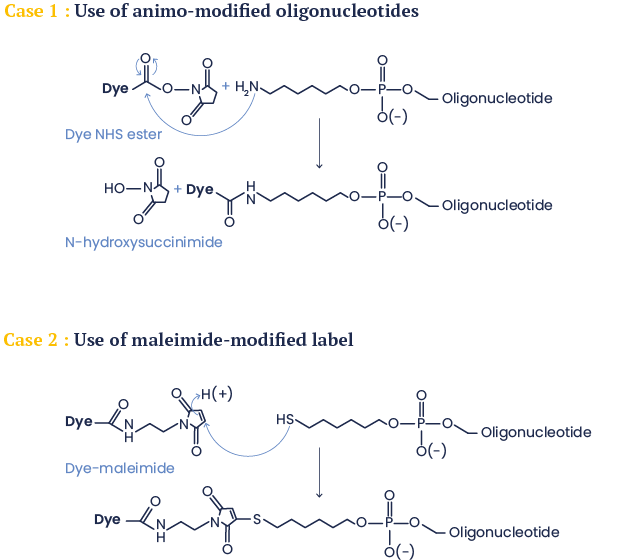
In the first case the label is conjugated to an amino-modified oligonucleotide (3’, 5’ or on a dT) using its amino-reactive version (N-hydroxysuccinimide (NHS) ester in most cases).
The second possibility (originally also used for synthesis of molecular beacons) is the addition of a maleimide-modified label to a thiol-modified oligonucleotide.