Tau peptides
Tau plays a key role in Alzheimer’s disease
Tau is a microtubule-associated protein that stabilizes and promotes microtubule assembly.
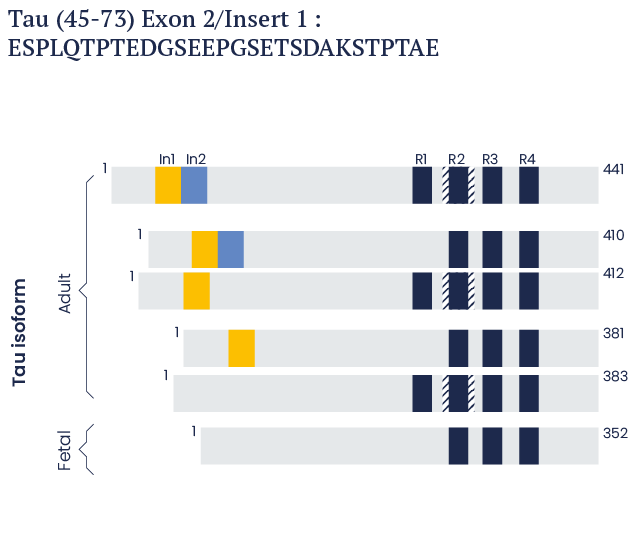
In the human brain, it is present as six isoforms that are 352, 381, 383, 410, 412, and 441 amino acids in length.1-5
When Tau is hyperphosphorylated, it can detach and form paired helical filament (PHF) aggregates.2-5 PHFs were found in neurofibrillary tangles in Alzheimer's disease (AD) patients. As such, hyperphosphorylation of Tau has been linked to AD.
In addition to being modified with phosphate groups, Tau can undergo glycosylation on the same regions where phosphorylation takes place.5 Discovery of this phenomenon has led to the hypothesis that glycosylation of Tau may help stabilizing microtubule-Tau connection and prevent free Tau aggregation.
References
1. Goedert, M. et al. Neuron, 3, 519 (1989).
2. Evans, DB. et al. JBC 275, 24977 (2000).
3. Luc, B. et al. Brain Res Rev 33, 95 (2000).
4. Arai, T. et al. JBC 280, 5145 (2005).
5. Yuzwa, SA. et al. Amino Acid 40, 857 (2011).
Human Tau isoforms
In the human brain, there are six TAU isoforms which are 352 to 441 amino acids long.
These isoforms vary at the carboxyl terminus according to the presence of either three repeat (3R) or four repeat (R4) domains, in addition to the presence or absence of one or two insert domains at the amino-terminus.
Shown beside is a schematic representation of Tau Peptide (45-73). This is a 29-amino acid long peptide derived from the Exon 2/Insert 1 domain.