COVID-19
SARS-CoV-2 serological antibody assays
Serological tests are useful tools for studying the evolution of the pandemic. We produce critical raw material peptides and antibodies that can be used to develop SARS-CoV-2 serological tests.
Why testing antibody production in patients?
The detection of antibodies produced in the host as a response to infection is suggested as a useful tool to evaluate past exposure to the virus. It is also an important information to determine how the antibody titer does or not correlate with viral protection.
Indeed, studies on Rhesus macaques showed that infection by SARS-CoV-2 generates a near-complete protection to reinfection (1), and limited cases of reinfection by SARS-CoV-2 have been evidenced in human. (2)
Detecting antibodies against SARS-CoV-2 is not useful for the diagnostic of COVID-19 but rather for a wide screening to estimate the prevalence and the fatality rate of coronavirus infection. Immunodiagnostic assays can also be a tool to determine if individuals can return to their social contacts. (3)
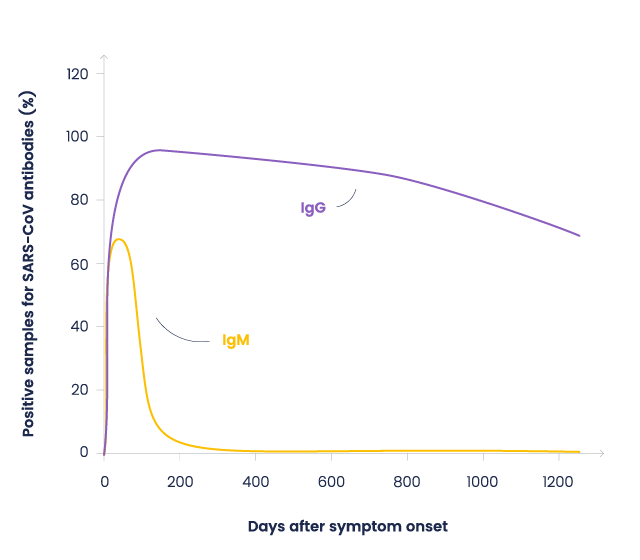
Previous studies on SARS-CoV showed that IgM appears after a week, followed by IgG production for a long-term (2-years) immunity (4). Firsts data about COVID-19 shows a similar pattern. (5)
Figure : Antibodies evolution after SARS-CoV-1 infection. Based on Wu, et al. data (4)
Coronavirus and anti-coronavirus antibody detection in blood by ELISA.
At the end of July 2020, more than 300 antibody-based tests were available.
Most of them are based on the Enzyme-Linked Immunosorbent Assay (ELISA) principle. ELISA is a high sensitivity and throughput test typically performed in a multi-well plate coated with antigen or antibodies. Detection is versatile as it can be colorimetric, fluorescent, chemiluminescent, or electrochemical.
Immunoassays can be used for:
Antigen tests: allowing the detection of the virus.
Serological tests: allowing the detection of host-derived antibodies against SARS-CoV-2 (3).
Antigen tests
Effective antigen-based tests could be an alternative to molecular testing. Viral antigens can be detected when the virus is actively replicating.
Antigen tests are rapid and highly specific but still may lack sensitivity. Thus, they can be used as a fast triage reducing the need for a molecular confirmation (3).
To improve diagnostic accuracy, one needs to develop and synthesize highly immunogenic, high-affinity viral antigens (3). Nowadays, several rapid test kits are under development or already available, i.e. using monoclonal antibodies against N protein of SARS-CoV-2(8).
Even if qPCR testing remains the method of choice for COVID-19 diagnostic, antigen testing is considered a good alternative to prevent the shortage of qPCR Reagents.
Principle
- Coating
Antibodies against SARS-CoV-2 protein are coated on a plate. - Biological samples from a patient potentially containing SARS-CoV-2 are added to the plate.
- Recognition
Labeled antibodies recognizing SARS-CoV-2-derived antigens are added to detect the presence of the virus in the sample. - Signal detection
Serological tests
Based on data on SARS-CoV, among the four structural proteins, Spike and Nucleocapsid proteins are suggested to be the most immunogenic (6,7). More precisely the S1 subunit of SARS-CoV-2 Spike protein is considered the most suitable antigen because it has the least overlap with other coronaviruses. (6)
Principle
- Coating
- Viral proteins or peptides are coated on a plate.
- Secondary antibodies recognizing human IgG or IgM are coated on a plate.
- Blood, plasma or serum, or potentially other biological fluids (like saliva) from a patient potentially containing anti-SARS-CoV-2 antibodies is added to the plate.
- Recognition
- Labeled secondary antibodies recognizing human IgG or IgM are added to detect the presence of anti-SARS-CoV-2 antibodies
- Labeled proteins or peptides are added to detect the presence of anti-SARS-CoV-2 antibodies
- Signal detection and antibody titer determination
Choose your weapon
We have various tools to help you in your serological test development
We have developed catalog peptide libraries derived from spike protein.
We also offer custom peptide library synthesis upon request.
| Catalog SARS-CoV-2 peptides libraries | Specifications |
|---|---|
| Custom peptides libraries | Learn more |
Besides ready-to-use catalog peptides from SARS-CoV-2 proteins including critical peptide domains/regions or peptide substrates, we can synthesize custom peptides from simple to highly complex sequences.
We also offer custom manufacturing of Critical Raw Material (CRM) peptides for demanding applications.
| Catalog SARS-CoV-2 peptides | Learn more |
|---|---|
| Custom peptide synthesis | Learn more |
| Critical raw material peptides | Learn more |
We are producing recombinant proteins like Nucleocapsid protein, Spike protein or a domain thereof, or ACE2 receptor variants, do not hesitate to contact us. Some of them are already available!
We are ready-to-start immunization programs targeting Spike glycoprotein from SARS-CoV-2 and other antigens.
SARS-CoV-2 related subjects
References
1) SARS-CoV-2 infection protects against rechallenge in rhesus macaques
Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, Tostanoski LH, Yu J, Maliga Z, Nekorchuk M, Busman-Sahay K, Terry M, Wrijil LM, Ducat S, Martinez DR, Atyeo C, Fischinger S, Burke JS, Slein MD, Pessaint L, Van Ry A, Greenhouse J, Taylor T, Blade K, Cook A, Finneyfrock B, Brown R, Teow E, Velasco J, Zahn R, Wegmann F, Abbink P, Bondzie EA, Dagotto G, Gebre MS, He X, Jacob-Dolan C, Kordana N, Li Z, Lifton MA, Mahrokhian SH, Maxfield LF, Nityanandam R, Nkolola JP, Schmidt AG, Miller AD, Baric RS, Alter G, Sorger PK, Estes JD, Andersen H, Lewis MG, Barouch DH.
Science. 2020 Aug 14;369(6505):812-817 - DOI: 10.1126/science.abc4776
2) COVID-19 and Postinfection Immunity: Limited Evidence, Many Remaining Questions.
Kirkcaldy RD, King BA, Brooks JT.
JAMA. 2020 Jun 9;323(22):2245-2246. DOI: 10.1001/jama.2020.7869
3) Molecular and Immunological Diagnostic Tests of COVID-19: Current Status and Challenges.
Kilic T, Weissleder R, Lee H.
iScience. 2020 Aug 21;23(8):101406 - DOI: 10.1016/j.isci.2020.101406.
4) Duration of antibody responses after severe acute respiratory syndrome.
Wu LP, Wang NC, Chang YH, Tian XY, Na DY, Zhang LY, Zheng L, Lan T, Wang LF, Liang GD.
Emerg Infect Dis. 2007 Oct;13(10):1562-4. - DOI: 10.3201/eid1310.070576.
5) A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients.
Liu L, Liu W, Zheng Y, Jiang X, Kou G, Ding J, Wang Q, Huang Q, Ding Y, Ni W, Wu W, Tang S, Tan L, Hu Z, Xu W, Zhang Y, Zhang B, Tang Z, Zhang X, Li H, Rao Z, Jiang H, Ren X, Wang S, Zheng S.
Microbes Infect. 2020 May-Jun;22(4-5):206-211. - DOI: 10.1016/j.micinf.2020.05.008
6) Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients.
Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q, Descamps D, Houhou-Fidouh N, Reusken CBEM, Bosch BJ, Drosten C, Koopmans MPG, Haagmans BL.
Emerg Infect Dis. 2020 Jul;26(7):1478-1488 - DOI: 10.3201/eid2607.200841
7) Validation of a commercially available SARS-CoV-2 serological immunoassay.
Meyer B, Torriani G, Yerly S, Mazza L, Calame A, Arm-Vernez I, Zimmer G, Agoritsas T, Stirnemann J, Spechbach H, Guessous I, Stringhini S, Pugin J, Roux-Lombard P, Fontao L, Siegrist CA, Eckerle I, Vuilleumier N, Kaiser L
Clin Microbiol Infect. 2020 Oct;26(10):1386-1394 - DOI: 10.1016/j.cmi.2020.06.024
8) Diagnostic Testing for Severe Acute Respiratory Syndrome-Related Coronavirus 2: A Narrative Review.
Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, Dittrich S, Yansouni CP.
Ann Intern Med. 2020 Jun 2;172(11):726-734 - DOI: 10.7326/M20-1301
9) Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections.
Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL.
Nat Med. 2020 Aug;26(8):1200-1204.
- DOI: 10.1038/s41591-020-0965-6
10) Will antibody tests for the coronavirus really change everything?
Mallapaty S.
Nature. 2020 Apr;580(7805):571-572.
- DOI: 10.1038/d41586-020-01115-z
11) False Negative Tests for SARS-CoV-2 Infection - Challenges and Implications.
Woloshin S, Patel N, Kesselheim AS.
N Engl J Med. 2020 Aug 6;383(6):e38.
- DOI: 10.1056/NEJMp2015897
12) Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection.
Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S.
J Virol. 2014 Oct;88(19):11034-44.
- DOI: 10.1128/JVI.01505-14.
13) Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study.
Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, Wang TB, Yang H, Richardus JH, Liu W, Cao WC.
J Immunol. 2011 Jun 15;186(12):7264-8
- DOI: 10.4049/jimmunol.0903490
14) Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients.
Yang LT, Peng H, Zhu ZL, Li G, Huang ZT, Zhao ZX, Koup RA, Bailer RT, Wu CY.
Clin Immunol. 2006 Aug;120(2):171-8.
- DOI: 10.1016/j.clim.2006.05.002.
15) SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls.
Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, Chng MHY, Lin M, Tan N, Linster M, Chia WN, Chen MI, Wang LF, Ooi EE, Kalimuddin S, Tambyah PA, Low JG, Tan YJ, Bertoletti A.
Nature. 2020 Aug;584(7821):457-462.
- DOI: 10.1038/s41586-020-2550-z.
16) Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19.
Sekine T, Perez-Potti A, Rivera-Ballesteros O, Strålin K, Gorin JB, Olsson A, Llewellyn-Lacey S, Kamal H, Bogdanovic G, Muschiol S, Wullimann DJ, Kammann T, Emgård J, Parrot T, Folkesson E; Karolinska COVID-19 Study Group, Rooyackers O, Eriksson LI, Henter JI, Sönnerborg A, Allander T, Albert J, Nielsen M, Klingström J, Gredmark-Russ S, Björkström NK, Sandberg JK, Price DA, Ljunggren HG, Aleman S, Buggert M.
Cell. 2020 Aug 14:S0092-8674(20)31008-4.
- DOI: 10.1016/j.cell.2020.08.017
17) Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals.
Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A.
Cell. 2020 Jun 25;181(7):1489-1501.e15.
- DOI: 10.1016/j.cell.2020.05.015
18) Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals.
Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H, Wei P, Ge J, Gou M, Li X, Sun L, Cao T, Wang P, Zhou C, Zhang R, Liang P, Guo H, Wang X, Qin CF, Chen F, Dong C.
Immunity. 2020 Jun 16;52(6):971-977.e3.
- DOI: 10.1016/j.immuni.2020.04.023.