COVID-19
COVID-19 vaccines
All around the world the scientists put a lot of effort to find efficient preventive and curative treatments for COVID-19. Several vaccine candidates are already in clinical trials. Among them, we can find the new generation of vaccines such as DNA vaccines and RNA vaccines.
COVID-19 Vaccines
Dozens of candidate vaccines against SARS-CoV-2 coronavirus are in pre-clinical and clinical evaluation.
Among them, we can find vaccines made of inactivated virus, protein subunits, virus-like particles, or vaccines using replicating or non-replicating viral vector technologies or gene-based vaccines such as DNA vaccines and RNA vaccines.
Nucleic acid vaccines and therapeutics are very promising molecules. They are particularly interesting when we consider pandemic evaluation treatments as multiple candidates can be designed and produced massively with a relatively faster time and reduced costs compared to classical protein-based treatments.
DNA vaccines
Potential COVID-19 DNA Vaccine candidates are under clinical evaluation.
DNA vaccines are based on the ability of small circular DNA molecules called plasmids
to produce antigens once inserted in the host cell nucleus.
DNA Vaccines efficiently stimulate humoral (antibody) and cellular (T cell) immune responses.
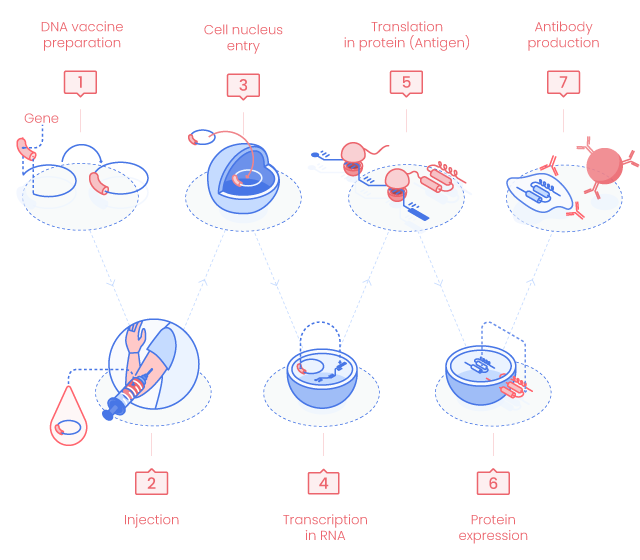
DNA Vaccine Principle
1. DNA vaccine preparation
DNA vaccines use highly active expression vectors (plasmid DNA, pDNA) optimized to generate a large amount of antigen in vivo and stimulate at best the host immune response (codon usage, promoter, polyadenylation…).
2. Delivery
DNA vaccines can be delivered by multiples methods but the first two are the main used ones.(1)
- Saline injection in muscles or skin
- Gene gun using compressed helium to deliver in the targeted cells gold or tungsten microparticles covered with pDNA
- Mucosal surface delivery using aerosol, biodegradable material, liposome preparation…
- Polymer vehicle
- Expression library immunization (ELI) to inject multiple genes of the pathogen at once.
3. Cell nucleus entry
pDNA enters the targeted cell nucleus where the genes carried on the pDNA will be processed as the host genes.
4. Gene transcription in RNA
pDNA genes are transcribed in mRNAs by the host cell enzymes. Generated mRNAs cross the nucleus membrane to reach the cytosol.
5. mRNA translation in proteins
mRNA is translated into peptides/proteins.
6. Antigen processing
Intracellularly produced proteins may be processed into small antigenic peptides by the host proteases.
In the endoplasmic reticulum (E.R.), peptides bind to MHC class I molecules to be presented on the cell surface in the context of the MHC I.
Proteins can also be presented by the MHC II pathway and initiate helper T cells (CD4+) responses.
7. Immune response
MHC class I/peptide complex stimulates CD8+ cytotoxic T cells (CTL). CTLs inhibit viruses through both cytolysis of infected cells and noncytolysis mechanisms such as cytokine production. (1)
MHC class II/antigen complex interacts with CD4+ T cells and activates immune response such as B cell stimulation followed by the production of specific antibodies. (2)
mRNA vaccines
mRNA vaccines are produced following a cell-free and scalable process.
With DNA vaccines, RNA vaccines constitute a good alternative when antigens are impossible to produce
with current expression systems and allow the complex proteins/antigens in vivo production.
As mRNA is a non-infectious, non-integrating platform,
there is no potential risk of infection or insertional mutagenesis.
Moreover, mRNA is degraded by normal cellular processes. (4)
Two main mRNAs are used for RNA vaccines
Non-replicating mRNA (NRM)
This mRNA contains a 5’ cap, a 5’ and 3’ untranslated regions (UTRs),
an open-reading frame (ORF) and a 3’ poly(A) tail
Self-amplifying mRNA (SAM)
This mRNA is identical to the NRM but contains in addition
the sequence coding for the viral replication machinery. (5)
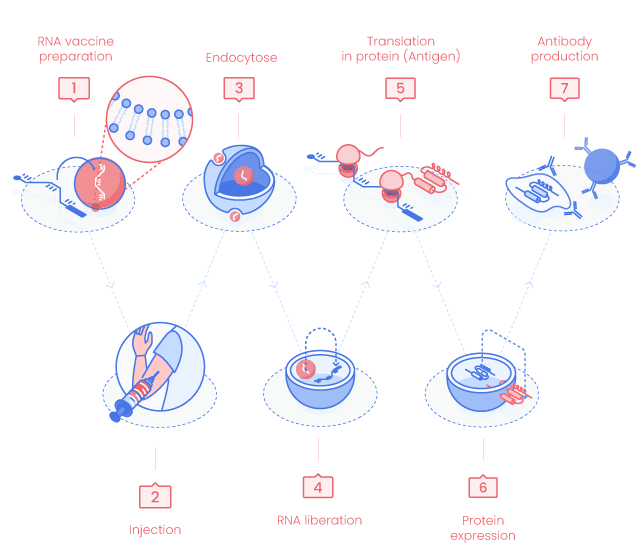
RNA Vaccine Principle
1. RNA vaccine preparation
mRNA coding for one or more antigens is enzymatically synthesized from DNA template such as PCR fragments, circular or linear plasmids.
2. Delivery
mRNA vaccine can be delivered by intramuscular or intradermal injection, using aerosol or directly into organs.
As naked RNA molecules are rapidly degraded by RNase, delivery reagents are optimized to facilitate entry in the host cells. mRNA vaccines can be injected as a naked RNA but also as cationic nanoemulsion, liposome, cationic polymers, cationic lipid nanoparticles and many other ways. (6)
3. Cellular uptake
mRNA enters the host cells. In this schematic representation, we consider the RNA in a liposome.
4. RNA liberation
RNA is released in the cytosol. mRNA does not enter the host cell nucleus and is degraded by normal cellular processes.
5. mRNA translation in proteins
mRNA is translated into peptide(s)/protein(s) using the host cell machinary.
6. Antigen processing
The generated protein(s) can be cleaved by proteases intracellularly in several peptides to constitute the antigens.
7. Immune Response
The produced antigens (peptides/proteins) will activate the innate and adaptative immune responses.
Vaccine development and manufacturing
We manufacture vaccines for clinical trial and commercial material for all major markets according to FDA, EMA, and PMDA requirements. We offer GMP biomanufacturing of Plasmid DNA, recombinant proteins, and protein conjugates. We also produce in vitro transcribed RNA (IVT-RNA) by enzymatic production.
Choose your weapons
We produce GMP Plasmid DNA for starting material, clinical trial, and commercial applications since 2004. We have significant experience and expertise in the large-scale production of plasmid DNA for various uses.
We offer process development and GMP manufacturing of In vitro transcribed RNA (IVT-RNA) API for use in Tox studies and Human clinical trials.
We develop processes and manufacture GMP recombinant proteins and chemically modified recombinant proteins for clinical trial and commercial uses.
All GMP material is produced in accordance to US, EU and Japanese regulatory requirements for sterile injectable products.
SARS-CoV-2 related subjects
References
1) DNA Vaccines.
Encke, J., Jasper zu Putlitz, and Jack R. Wands. 1999.
Intervirology 1999;42:117-124 – DOI : 1159/000024971
2) Revealing the Potential of DNA-based Vaccination: Lessons Learned from the Hepatitis B Virus Surface Antigen.
Schirmbeck, R. and Jorg Reimann. April 2001.
Biol. Chem., 382:543-552. – DOI : 10.1515/BC.2001.068
3) Immunogenicity of a DNA vaccine candidate for COVID-19
Smith et al.;
NATURE COMMUNICATIONS | (2020) 11:2601 - DOI : 10.1038/s41467-020-16505-0
4) mRNA vaccines — a new era in vaccinology.
Norbert Pardi, Michael J. Hogan, Frederick W. Porter, and Drew Weissman.
Nat Rev Drug Discov. 2018 April ; 17(4): 261–279 – DOI : 10.1038/nrd.2017.243.
5) The promise of mRNA vaccines: a biotech and industrial perspective.
Nicholas A. C. Jackson, Kent E. Kester, Danilo Casimiro, Sanjay Gurunathan and Frank DeRosa.
npj Vaccines (2020) 5:11 - DOI : 10.1038/s41541-020-0159-8
6) mRNA vaccines — a new era in vaccinology.
Norbert Pardi, Michael J. Hogan, Frederick W. Porter, and Drew Weissman.
Nat Rev Drug Discov. 2018 April ; 17(4): 261–279 – DOI : 10.1038/nrd.2017.243.