COVID-19
COVID-19 molecular diagnostic tests
The absence of specific symptoms for COVID-19 calls for very accurate diagnosis methods. Get here an insight to the most common use methods.
Diagnostic tests for coronavirus detection
SARS-CoV-2, the virus responsible for COVID-19 pandemic, requires ACE2 receptor to enter the human cells. (1)
ACE2 is a cellular receptor highly spread over various cell type in human body. This wide distribution may explain SARS-CoV-2 can penetrate and damage various organs (lungs, kidney, intestine, brain…).
COVID-19 symptoms are various and may overlap with common cold. In some patients, COVID-19 can go undetected (asymptomatic infection) while in others COVID-19 may be fatal.
The absence of specific symptoms calls for very accurate diagnosis methods. Ideally, the testing phase should be done massively and deliver results in the quickest time to isolate COVID patients and control the spread of the contamination.
Various diagnostic methods based on viral RNA, viral antigens, or specific antibody detection in host samples are available on the market. (2,3) While the RNA- and viral antigen-based methods indicate a current infection, the antibody detection brings information about a previous contamination.
Viral RNA detection
2019-nCoV RNA analysis is realized by Nucleic acid amplification tests using primer and probe oligonucleotides targeting approved sequences of the coronavirus.
Positive detection in individual specimen samples indicates an active infection.
Viral antigen detection
The most common target antigens from the SARS-CoV-2 virus is the nucleocapsid (N) protein and spike (S) protein.
N and S proteins from the respiratory tract can be detected using capture antibodies via Lateral Flow Assay (LFA) and ELISA.
Specific antibody detection
Serological tests detect in blood anti-SARS-CoV-2 IgG and IgM.
Testing can be performed by LFA and ELISA using capture agents such as SARS-CoV-2 peptides.
Positive results indicate that the patient has been exposed to the virus
RT-qPCR Assays
RT-qPCR is the Gold standard to detect foreign nucleic acids in patient samples. This is a very sensitive and fast method enabling the detection of the virus even a few days after infection.
In the case of SARS-CoV-2 infection, it has been shown that viral concentration in the upper respiratory tract is higher than in other body parts.
Accordingly, samples from the nasopharynx are recommended for COVID-19 diagnosis.
WHO (World human organization) and cdc (Centers for Disease Control and Prevention) published their own RT-PCR method for COVID-19 detection but the general process is globally identical.
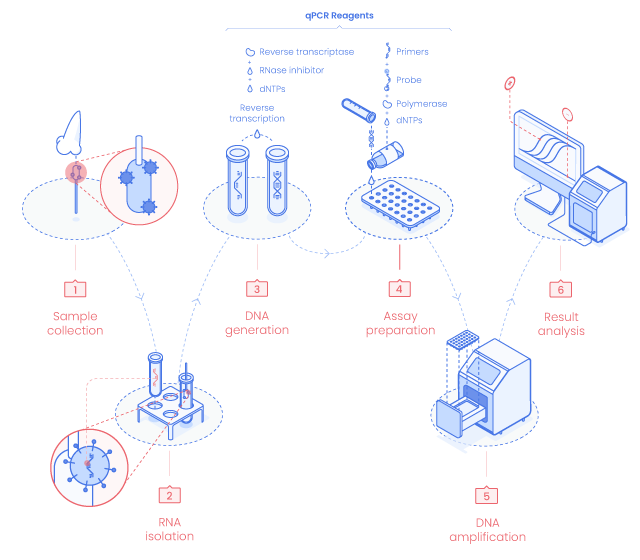
RT-qPCR testing principle
Sampling & RNA Extraction
The detection process starts with an RNA extraction from the sample collected. Sampling is performed with a swab kept refrigerated in a sterile tube containing viral transport medium after sample collection. In those conditions, specimens remain stable for up to 72 h after collection. (2)
Reverse Transcription : cDNA generation
The RNA extract is mixed with SARS-CoV-2 specific primers, probe, enzymes, and other components to generate cDNA by reverse transcription of the virus RNA present in the sample.
Reverse transcription and DNA amplification are usually performed in a single-tube “One-Step” approach to ensure test rapidity and reduce contamination risk.
PCR Cycles : DNA Amplification
Virus cDNA is then amplified by PCR cycles. Genetic material amplification is recorded by measuring the fluorescence intensity generated by the qPCR Probe. The more the amount of target cDNA is present, the stronger the fluorescent signal becomes.
The Probe binds to a specific sequence on the cDNA located between the primers annealing sites. As the primers and qPCR Probe are specific to the SARS-CoV-2 cDNA, the fluorescent signal will only be emitted in samples containing SARS-CoV-2 genetic material. Primer and Probe sequences for SARS-CoV-2 detection have been published by cdc and WHO.
Result Interpretation
A cycle threshold (Cq) value less than 40 is usually considered as positive. Others consider a Cq of 35 as the upper limit representing an active, transmissible infection. Samples from healthy patients containing no SARS-CoV-2 genetic material, will not be amplified and result in a negative response.
Positive and negative internal controls are also tested in parallel to avoid false negative and positive results respectively.
Saliva testing
A publication made in September 2020 suggest that Saliva specimens can also be used to detect coronavirus infection by amplification reactions. This sampling method gives similar sensitivity than nasopharyngeal swab specimens. Recent studies also shown that the virus could be detected in saliva samples from asymptomatic people.
This method of sampling could bring several considerable advantages as the collection of saliva is made by the patients themselves:
- Avoid bottlenecks at testing centers,
- No interaction between the patients and the collector
- Reduce amount of supplies (swabs).(4)
Products to diagnose COVID-19 patients
As a biotechnology company delivering reagents for research, diagnostic and therapeutic usage, we are concerned by this sanitary crisis and want even more than ever to support the Life science and medical community in the fight against the COVID pandemic.
We have stepped up efforts to actively produce primers, probes, and other RT-qPCR reagents and ensure continuity of supply all along with this health crisis. We support and help our customers to reach their daily testing goals.
Choose your weapons
As a primary manufacturer of amplification reagents, Eurogentec is supporting the local and international efforts against the COVID pandemic. Our unique One-Step Takyon™ RT-qPCR reagents are actively used by several leading European labs for the diagnosis of COVID patients.
We also developed a unique reverse transcription qPCR kit optimized for sensitive and specific SARS-CoV-2 RNA detection with increased multiplex capabilities.
| One-Step Takyon™ RT-qPCR optimized for "COVID-19" | Evaluate |
|---|
We synthesize oligonucleotides for research, diagnostic, and therapeutic applications.
We are experts in the production of primers, complex oligonucleotides and Probes and have a large portfolio of usual and exotic modifications including Access™ Dyes and Quenchers, a class of proprietary molecules or others dyes & quenchers offering unmatched detection performance and IP-friendly terms and conditions.
| RUO Oligonucleotides | Configure |
|---|---|
| Diagnostic Oligonucleotides | Request a Quote |
| RUO Double-Dye Probes | Configure |
| Molecular Beacons | Configure |
| MGB Probes | Configure |
| Access™ Dyes and Quenchers | View our offer |
In addition to the Takyon™ DNA polymerase included in our qPCR and RT-qPCR kits, we offer individual research and IVD grade enzymes for nucleic acid amplification. Our portfolio includes classical, HotStart and high sensitivity enzymes.
We offer various qPCR Controls to guarantee the integrity of your amplification reaction from the nucleic acid extraction phase to the result interpretation.
| Housekeeping controls | Shop Now |
|---|---|
| Custom housekeeping controls | Request a quote |
| Internal positive controls | Shop Now |
| Sample processing controls | Shop Now |
We offer a highly flexible and custom service for the preparation and the delivery of your Lab Developed Tests (LDTs) in a ready-to-use format.
SARS-CoV-2 related pages
References
1) A pneumonia outbreak associated with a new coronavirus of probable bat origin.
P. Zhou, X.L. Yang, X.G. Wang, B. Hu, L. Zhang, W. Zhang, H.R. Si, Y. Zhu, B. Li, C.L. Huang, H.D. Chen, J. Chen, Y. Luo, H. Guo, R.D. Jiang, M.Q. Liu, Y. Chen, X.R. Shen, X. Wang, X.S. Zheng, K. Zhao, Q.J. Chen, F. Deng, L.L. Liu, B. Yan, F.X. Zhan, Y.Y. Wang, G.F. Xiao, Z.L. Shi.
Nature 579 (7798) (2020) 270–273 – DOI: 10.1038/s41586-020-2012-7
2) Molecular and Immunological Diagnostic Tests of COVID-19: Current Status and Challenges.
Tugba Kilic, Ralph Weissleder, and Hakho Lee.
iScience 23, 101406, August 21, 2020 - DOI : 10.1016/j.isci.2020.101406
3) COVID-19 diagnostics in context.
Weissleder, R., Lee, H., Ko, J., and Pittet, M.J.
Sci. Transl. Med. 12, 546, eabc1931. June 03, 2020 - DOI: 10.1126/scitranslmed.abc1931
4) Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2
n engl j med 383;13. September 24, 2020 - DOI: 10.1056/NEJMc2016359